Introduction:
Currently, lithium-ion batteries are facing challenges such as a global shortage of lithium resources, high production costs and the insecurity of organic electrolytes, which limit their further development, especially in large-scale energy storage systems. Rechargeable aqueous zinc-ion battery (ZIBs) uses water as solvent, has the advantages of low cost, high operation safety and environmental friendliness, and has huge potential in the application of large-scale energy storage system.
As the anode of zinc battery, metal zinc has low cost and is easy to be produced on a large scale. However, the zinc anode suffers uncontrollable dendrite growth and electrolyte corrosion, resulting in poor reversibility. The researchers hope to develop strategies to protect the Zn anode to inhibit dendrite growth. Recently, various strategies have been developed to inhibit the growth of zinc dendrites, but the relationship between the crystallization behavior and deposition morphology during zinc electrodeposition has seldom been studied.
Recently, Prof. Hongbin Lu of the Department of Polymer Science, Fudan University, Prof. Jia Guo of Fudan University, Prof. Chengxin Peng of The University of Shanghai for Science and Technology, and Prof. Zaiping Guo of the University of Adelaide, Australia, reported a multifunctional platform based on two-dimensional (2D) covalent organic framework (COF). An ultrathin, porous and fluorinated COF (FCOF) film with high mechanical strength has been successfully developed as a protective layer (FCOF@Zn) on the zinc anode surface. The composite anode achieves the horizontal deposition morphology of zinc sheet and eliminates dendrite growth. At the same time, the fluorinated nano channel is conducive to accelerating ion conduction, limiting water penetration, alleviating the corrosion of zinc anode by electrolyte, and greatly prolonging the cycle life of zinc anode. In this paper, "Horizontally arranged zinc electrodeposit modulated by Fluorinated Covalent Organic framework film for platochrome platozinc platoplasma High-rate and durable aqueous zinc ion batteries. "Nature Communications, 16 November 2021. The first authors of this paper are Zedong Zhao, PhD candidate, department of Polymer Science, and Rong Wang, PhD. The paper links:https://doi.org/10.1038/s41467-021-26947-9

Key points:
1) The research team prepared iminated FCOF films by solvothermal method. In a typical process, two monomers (2, 3, 5, 6-tetrafluoro-p-benzaldehyde (TFTA) and 1, 3, 5-tri (4-aminophenyl) benzene (TAPB)) are dissolved in a dioxane/mesitylene (D/M) mixture, followed by condensation polymerization using acetic acid as catalyst in a solvent heat pipe.
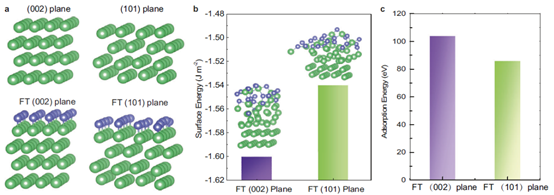
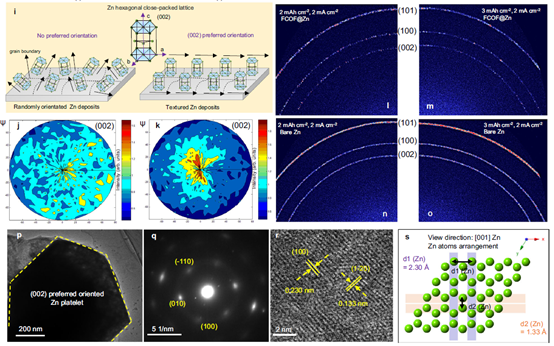
2) From the perspective of surface adjustment of Zn crystal, a large number of F atoms are introduced into FCOF film. The electronegative F atom has a strong interaction with its underlying Zn atom, resulting in a lower surface energy on Zn(002) surface than on conventional Zn(101) surface. Therefore, Zn deposition presents a sheet structure with preferred orientation along (002) plane, and the sheet structures are parallel to each other to form a planar zinc deposition structure. In addition, FCOF films are continuous and dense, and have a strong bond with zinc, remaining intact on the zinc surface and providing lasting protection. At the same time, 2D stacking and covalent bonding make the films have excellent mechanical properties. The film has an elastic modulus of more than 30 GPa, which can buffer the bulk expansion of Zn during the cycle. Finally, FCOF films have ultra-light mass, ultra-thin thickness (100 nm) and can be precisely adjusted at the nanoscale without affecting the mass or volumetric energy density of the zinc anode.
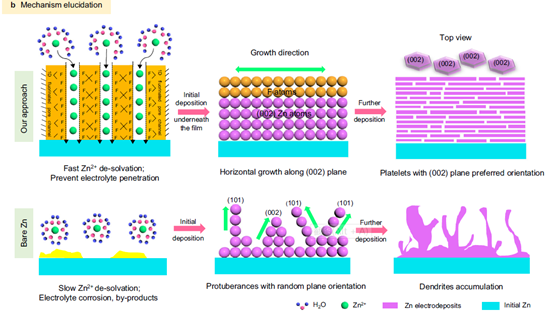
3) By means of experimental tests combined with theoretical calculations, the team members discussed in detail the formation mechanism of the deposition morphology of horizontally arranged zinc flakes from the perspective of crystallography.
4) The experimental results show that FCOF@Zn anode has a long cycle life and good reversibility in the range of large current density (5-80 mA cm-2). At ultra-high current density of 40 mA cm-2, the stability of the FCOF@Zn symmetric cell exceeds 750 h, far exceeding the performance reported in previous literature. In addition, the full battery assembled by pairing with manganese dioxide (MnO2) positive electrode has a stable cycle life of more than 250 times under the practical conditions of poor electrolyte, high area capacity positive electrode and limited zinc, showing a very competitive commercial prospect.