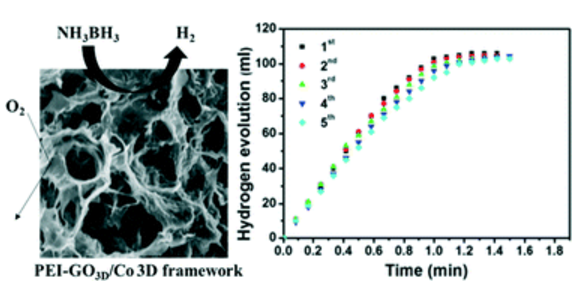
Low-cost, stable and highly efficient catalysts are essential for practical energy applications. In this study, branched polyethylenimine (PEI)-decorated graphene oxide (GO) 3D structures were prepared through a hydrothermal reaction. Cobalt (Co) nanoparticles (NPs) were deposited on the PEI-GO 3D structures, forming the desired, low-cost composite catalyst PEI–GO3D/Co. The catalyst shows a quite high catalytic activity for the room temperature hydrolysis of ammonia borane, with a turnover frequency of 18.5 molH2min−1molCo−1and a hydrogen generation rate of 7.68 LH2min−1gCo−1. The activation energy of the catalyst is relatively low (27.41 kJ mol−1), along with an ultrahigh cycle stability, that is, ∼83% of the initial catalytic activity was retained after 5 catalytic cycles, superior to the majority of non-noble metal catalysts.
This work was published on Catal. Sci. Technol., see details: Mengxiong Li, Jiantong Hu and Hongbin Lu*, A Stable and Efficient 3D Cobalt-Graphene Composite Catalyst for the Hydrolysis of Ammonia Borane,Catal. Sci. Technol., 2016, DOI: 10.1039/c6cy01253a.